Virtual Screening Reveals New Inhibitors for an Inflammation-Related Enzyme
Researchers from the RIDC Redoxoma have identified novel inhibitors of the human 15-lipoxygenase-2 (h15-LOX-2) enzyme using virtual screening techniques. This enzyme plays a critical role in inflammatory and metabolic processes and maintaining cellular homeostasis. The discovery of h15-LOX-2 inhibitors may open new avenues for investigating the enzyme’s biological and pathological roles and provide promising candidates for drug development.
“Although h15-LOX-2 has a potential role as a biological target, it has been little explored for this purpose. Our work contributes to new inhibitors with structural diversity among themselves and in relation to inhibitors already described in the literature. And, in addition, they present drug-like properties according to predictions based on computational models,” said Lucas G. Viviani, lead author of the article published in the Journal of Medicinal Chemistry. Viviani is a postdoctoral fellow at the Laboratory of Modified Lipids and Redox Biochemistry at the Instituto de Química, USP, under the guidance of Professor Sayuri Miyamoto.
h15-LOX-2 belongs to the family of lipoxygenases (LOXs), enzymes that catalyze the oxidation of polyunsaturated fatty acids forming specific hydroperoxides. In humans, six LOX isoforms have specialized roles in different tissues, regulating processes such as inflammation, cell proliferation, and regulation of the intracellular redox state. h15-LOX-2 is predominantly expressed in macrophages, skin, cornea, lungs, and prostate, where it catalyzes the conversion of arachidonic acid into a compound that significantly influences inflammatory and cellular responses.
“In the inflammatory cascade, h15-LOX-2 is one of the few enzymes capable of acting on complex lipids. It can oxidize membranes and cholesterol esters, whereas most lipoxygenases act on free fatty acids. We chose to study this enzyme also because of its unique enzymatic activity,” said Miyamoto.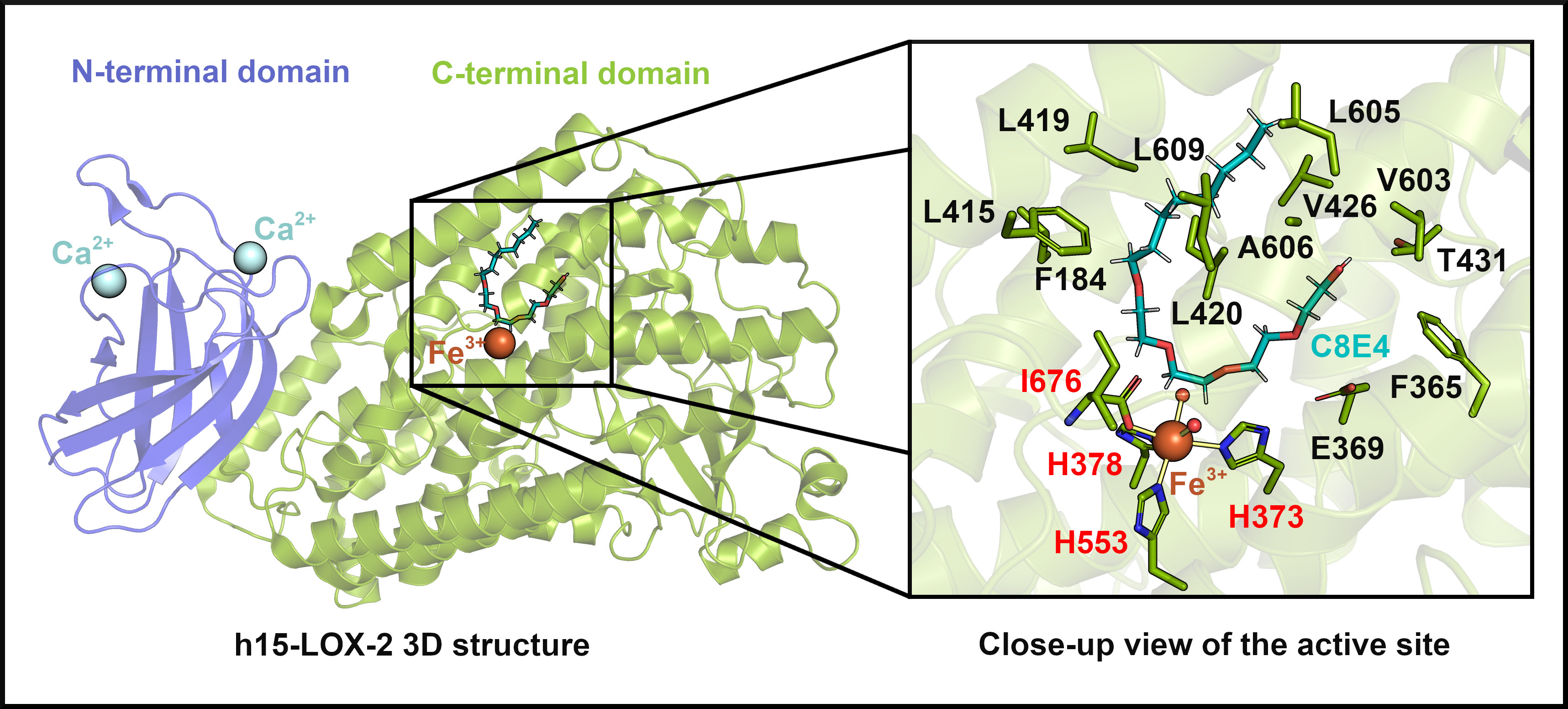
Virtual screening and experimental validation
Virtual screening is a technique that employs computational methods to select compounds with potential biological activity from large databases. In this study, the researchers began with a library of eight million compounds prefiltered for drug-like properties, considering factors such as absorption, distribution, metabolism, excretion, and toxicity. They then applied sequential filters based on different methodological approaches to simplify the process.
“A key aspect of this work was the use of a molecular shape-based similarity filter as the first step, based on the idea that there is a shape complementarity between a low molecular weight compound - such as an inhibitor - and its binding site in the target protein. An advantage of this method is that even compounds that share the same shape can have different structures. Since one of our goals was to select compounds structurally different from the existing inhibitors, this step was fundamental to the success of our approach,” explained Viviani.
The second filter prioritized structural dissimilarity, further enhancing the diversity of the selected compounds. Structural diversity is crucial for drug development because many steps must be considered after discovering an inhibitor. Starting with a broader range of structures increases the likelihood of successfully developing a drug.
After this step, the scientists used the docking method, which simulated how the compounds bind to the enzyme’s active site and analyzed the results by visual inspection. At the end of the virtual screening, 39 compounds were identified as potential h15-LOX-2 inhibitors. Of these, 14 were subjected to enzymatic assays to evaluate their inhibitory activity, and six showed promise. Ultimately, two inhibitors were identified as the most potent and selected for further optimization.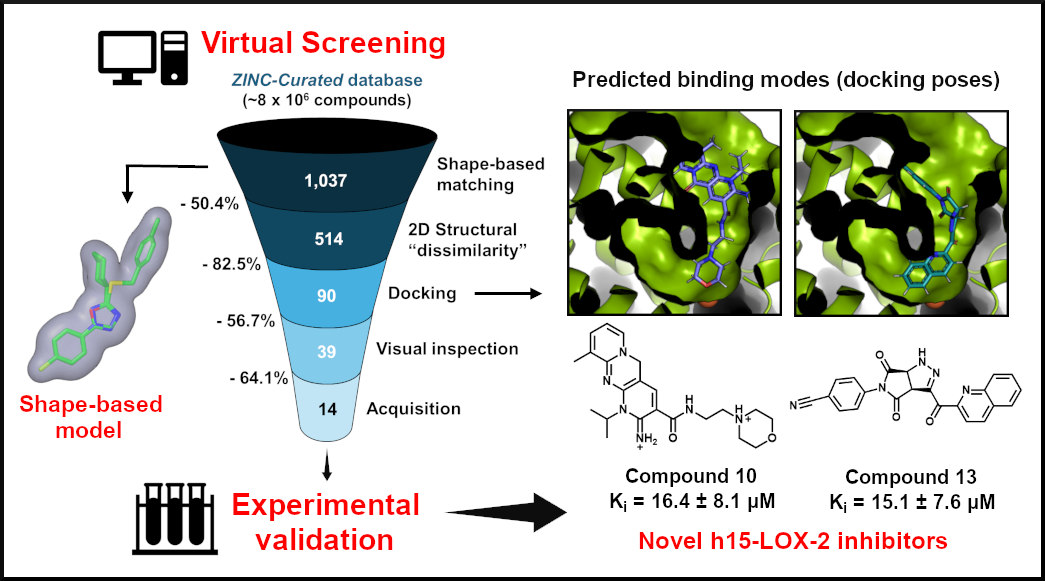
“What surprised us was to observe experimentally that these two compounds had a mixed type of inhibition mechanism. This means that, possibly, these compounds, in addition to binding to free enzyme, could also bind to the enzyme-substrate complex”, Viviani said.
To perform in vitro enzymatic assays and experimentally validate the virtual screening results, the researchers expressed and purified h15-LOX-15 enzyme in collaboration with the group of researcher Luis Netto, from the Instituto de Biociências at USP and a member of the RIDC Redoxoma.
Physiological and pathological roles
The specific physiological roles of h15-LOX-2 remain under investigation. The enzyme plays a key role in the biosynthesis of inflammatory lipid mediators and the development of atherosclerotic plaques. Research has shown that its expression is significantly elevated in atherosclerotic lesions of the human carotid artery compared to healthy arteries. Furthermore, evidence suggests a potential link between h15-LOX-2 and the development of certain types of cancer.
In prostate cancer, for example, the enzyme appears to suppress tumor formation. “Studies show that in healthy prostate cells, h15-LOX-2 is constitutively expressed, whereas in tumor cells, its expression is reduced. There is a lot of evidence pointing to a tumor suppressor role for this enzyme,” said Thais S. Iijima, master’s student and co-first author of the article.
Furthermore, h15-LOX-2 and other LOX enzymes may be involved in ferroptosis, an iron-dependent form of cell death associated with lipid peroxidation. In this case, enzyme inhibition could be beneficial.
The enzyme may also be related to the regulation of cellular senescence in epithelial cells and play a role in cholesterol homeostasis in macrophages, as described in recent studies.
“In general, there are few studies on h15-LOX-2. Inhibitors are important because they can be used to manipulate enzyme activity in cells, which would help to better understand its biological role,” Miyamoto said.
Next steps
Now, the researchers plan to propose structural changes to the identified compounds to increase their inhibitory potency. “Another aim is to improve some physicochemical properties, such as solubility in aqueous media, which might favor their pharmacokinetic properties,” said Viviani.
Miyamoto emphasized that further testing is needed. “In addition to improving efficiency, the inhibitors need to be tested in cells and animal models to confirm they reach the target enzyme and act as intended.“
The article Identification of Novel Human 15-Lipoxygenase-2 (h15-LOX-2) Inhibitors Using a Virtual Screening Approach, by Lucas G. Viviani, Thais S. Iijima, Erika Piccirillo, Leandro Rezende, Thiago G. P. Alegria, Luis Eduardo S. Netto, Antonia T.-do Amaral and Sayuri Miyamoto, can be accessed here.
