Scientists unravel bacterial resistance mechanism involving vitamin C
Throughout evolution, pathogenic microorganisms including bacteria, viruses, and fungi, have developed sophisticated defense strategies to survive and proliferate within their hosts’ hostile environment. These mechanisms enhance virulence and make infections more difficult to combat. One of the most effective strategies is the neutralization of oxidants released by immune cells to eliminate invaders.
In a recent article published in Redox Biology, a research group led by Luis E. S. Netto, from the Instituto de Biociências at the University of São Paulo (USP) and a member of the Research Center for Redox Processes in Biomedicine (RIDC Redoxoma), describes how the protein LsfA, a 1-Cys peroxiredoxin, protects the bacterium Pseudomonas aeruginosa against hydrogen peroxide produced during the immune response. LsfA catalyzes the elimination of hydroperoxides using ascorbate (vitamin C) as a reducing agent, thereby reinforcing the bacterium’s antioxidant defense.
“A major contribution of our work is the structural determination of a protein involved in the virulence of a medically important bacterium. We also demonstrated that ascorbate can function as a reductant in a cellular system, which is quite novel. In technical terms, we were the first to use the HyPer7 probe in Pseudomonas,” said Rogério L. Aleixo-Silva, first author of the article. He conducted the research during his master’s and doctoral studies at the Laboratory of Proteins and Redox Biology at IB-USP and is currently a postdoctoral fellow at the University of Massachusetts Chan Medical School in the United States.
The novel structural data obtained in this study may open new opportunities for the development of specific inhibitors of the bacterial enzyme, driving the advancement of new therapeutic strategies.
“This is the first study with the biochemical and structural characterization of a bacterial Prx6. In the literature, among the three major domains of life — Eubacteria, Archaea, and Eukarya — there are already many resolved structures of Prx6, mainly in archaea and mammals, including humans. But this is the first structure of a bacterial Prx6. E coli, a classic bacteria model, does not have Prx6,” said Luis Netto.
Antioxidant system
Pseudomonas aeruginosa is an opportunistic bacterium that causes infections mainly in people with weakened immune systems. It is responsible for several types of hospital-acquired infections, including pneumonia in patients with cystic fibrosis, urinary tract infections, infections in burns and surgical wounds, as well as endocarditis and septicemia. The World Health Organization (WHO) lists it as a priority bacterial pathogen for the development of new treatments due to its high resistance to antibiotics.
When invaded by a pathogen, the organism reacts by mobilizing its immune defenses, such as phagocytes, cells that fight microorganisms by releasing reactive oxygen, nitrogen, and chlorine species. In response to this oxidative stress, bacteria such as P. aeruginosa activate protective mechanisms that involve several antioxidant proteins, including peroxiredoxins (Prxs).
LsfA is a peroxiredoxin of the Prx6 subfamily present in Pseudomonas aeruginosa and is associated with bacterial virulence. Previous studies led by researcher Regina Baldini, from the rom the Departamento de Bioquímica at IQ-USP, had already demonstrated its importance in infection models using macrophages and mice. In the new study, researchers delved deeper into the molecular mechanisms underlying its protective function, showing that, although P. aeruginosa harbors an arsenal of antioxidant enzymes, LsfA stands out due to its high efficiency in decomposing hydrogen peroxide.
A central finding of the study is the interaction between LsfA and vitamin C (ascorbate). In 2007, Netto’s group showed that ascorbate can reduce the sulfenic acid formed in the oxidation of 1-Cys peroxiredoxins, challenging the prevailing view that these enzymes depend exclusively on recycling by thiols. The study, published in PNAS, revealed a new function of vitamin C as an intracellular reducing agent.
Although the role of ascorbate as a Prx reductant is still not fully understood in biological systems, structural analyses conducted in this study suggest that ascorbate interacts directly with LsfA’s active site. This interaction enables the enzyme to be regenerated after oxidation, thereby restoring its antioxidant function.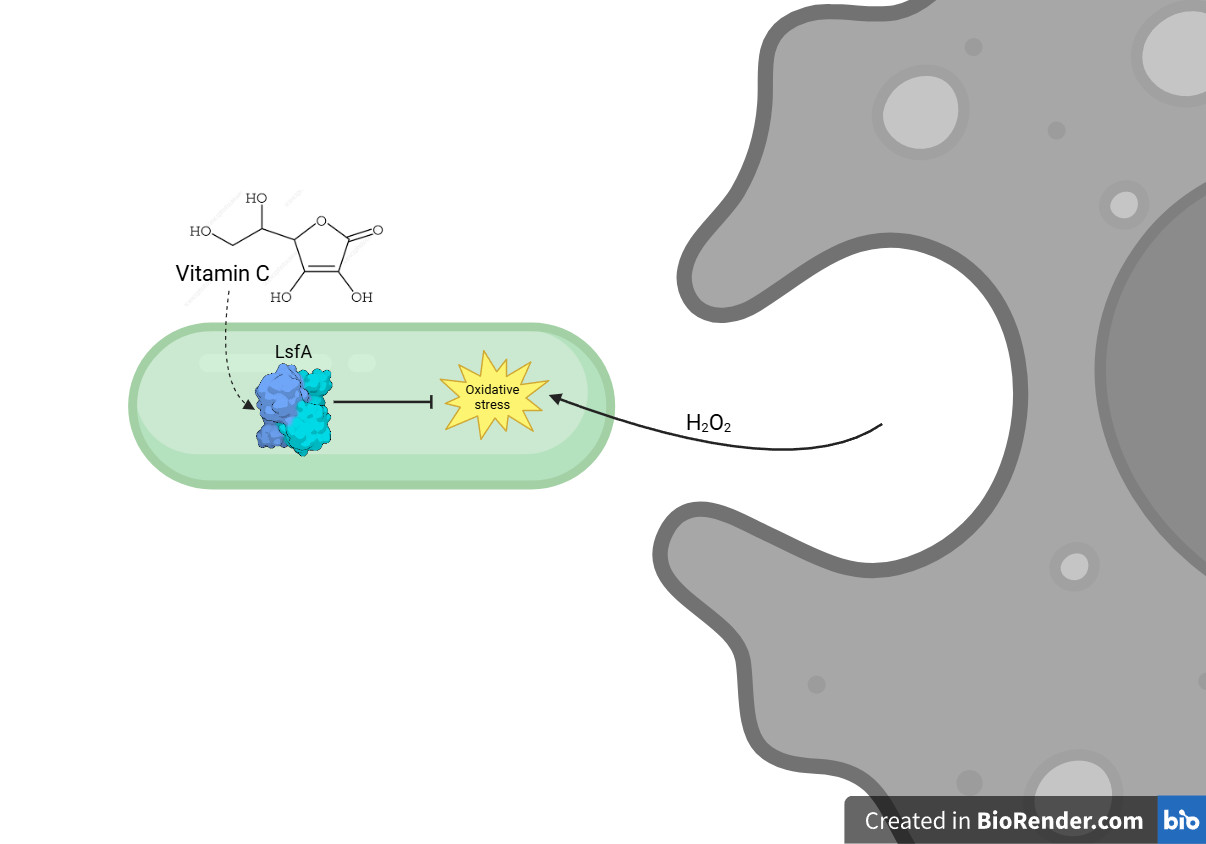
Experiments using the HyPer7 probe helped confirm the presence of ascorbate inside bacterial cells. HyPer7 is a fluorescent probe based on the green fluorescent protein (GFP), whose brightness changes in the presence of hydrogen peroxide, allowing the detection of this molecule inside living cells in real time. The results obtained with the expression of HyPer7 in P. aeruginosa showed that hydrogen peroxide reduction is more efficient in the presence of LsfA and ascorbate
“We know that Pseudomonas has no known biosynthetic pathways to synthesize ascorbate, nor have any identified transport proteins for its uptake. Yet, with the probe, we were able to show not only that LsfA activity depends on the presence of ascorbate, but also, in a sense, that ascorbate is indeed entering the bacterial cell,” Aleixo-Silva said. The researchers propose Pseudomonas may internalize ascorbate from immune cells.
The role of LsfA in P. aeruginosa’s response to oxidative stress was investigated using a genetically modified bacterial strain in which the lsfA gene was deleted (ΔlsfA strain). The researchers combined classical methods, such as counting colony-forming units after exposure to hydrogen peroxide, with real-time detection of the oxidant using the HyPer7 fluorescent probe. “We observed that in the absence of LsfA, the bacteria became more sensitive to peroxide and less able to neutralize it,” the researcher explained.
Inhibitor
Because bacterial LsfA has a human homolog, any potential inhibitor must selectively target the bacterial form without affecting the human one. The researchers showed that, although structurally similar to other Prx6 proteins, bacterial LsfA exhibits distinct electrostatic properties, meaning that the active sites have different charges. These differences influence the way an inhibitor would interact with each version of the enzyme.
“The advantage of our paper is that, in addition to resolving the structure, we used in silico docking to show some of the interactions between LsfA and ascorbate that could potentially be mimicked by an inhibitor,” said Aleixo-Silva.
According to Netto, the next steps include deeper investigation into ascorbate metabolism in P. aeruginosa, as well as studies using macrophage models to assess how LsfA deletion affects both bacterial defense and host inflammatory responses - a line of inquiry already supported by preliminary, unpublished lipidomics data.
The article Interaction between 1-Cys peroxiredoxin and ascorbate in the response to H2O2 exposure in Pseudomonas aeruginosa, by Rogerio L. Aleixo-Silva, Renato M. Domingos, Madia Trujillo, Fernando Gomes, Luciene O. Machado, Cristiano L.P. Oliveira, Regina Baldini, and Luis E.S. Netto, can be accessed here.
