Scientists propose updating textbooks after a study highlights the role of sodium in mitochondrial energy transformations
Transformations of energy vital to life occur primarily in mitochondria, the center of energy metabolism. The body’s “energy currency”, adenosine triphosphate (ATP), is generated in these organelles through a process known as oxidative phosphorylation. Remarkably, the average person synthesizes an amount of ATP equivalent to their body weight daily.
Oxidative phosphorylation is the energy-transfer process involving electrical and proton gradients across the inner mitochondrial membrane. This mechanism links coupling the gradual oxidation of electron donors in the electron transport chain with the pumping of protons across the membrane, generating the electrochemical gradient necessary for ATP synthesis. In some ways, mitochondria are similar to batteries.
Recent research into the location of the electron transport chain in mitochondria and the role of sodium in mitochondrial respiration has led Alicia Kowaltowski, a researcher at the Instituto de Química at Universidade de São Paulo (USP) and a member of RIDC Redoxoma, to advocate for updating textbooks. In collaboration with Fernando Abdulkader, she published an article titled “Textbook Oxidative Phosphorylation Needs to Be Rewritten”, in the journal Trends in Biochemical Sciences. The article discusses a few recent important discoveries about mechanisms in oxidative phosphorylation, including a groundbreaking study published in Cell by Spanish researcher José Antonio Enríquez and his team, revealing sodium’s unexpected role in maintaining mitochondrial membrane potentials.
“Knowledge evolves, and so must what is presented to students,” Kowaltowski explained. “Until a few years ago, we were certain that mitochondria produced ATP through oxidative phosphorylation in the space where the inner and outer membranes interact. That changed because we discovered that this process occurs inside the mitochondrial cristae. The textbooks are wrong and it is time to change that. Now with the work of Enríquez’s group, we discovered that the property of the membrane potential can also be a little different, an aspect not currently covered in textbooks.“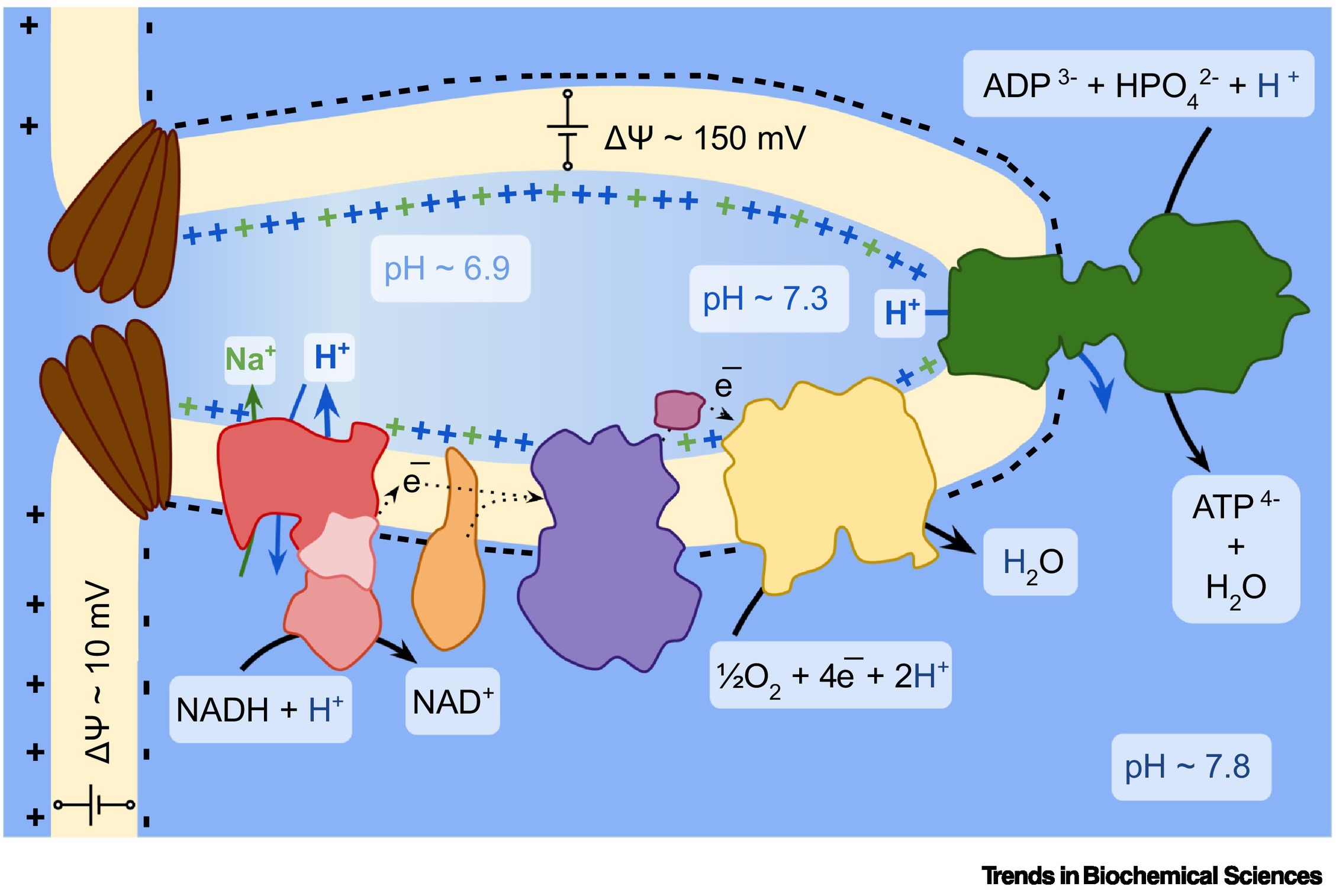
Sodium and membrane potential
It has long been known that the proton gradient (ΔpH) in mitochondria is small due to cellular buffering mechanisms that stabilize the pH. Therefore, the charge gradient (Δψ) has been considered the dominant component of the proton pumping. Until recently, this gradient was attributed to potassium, the most abundant cation in cells.
However, the study by Enríquez’s group revealed that between 30% and 50% of this gradient can be attributed to sodium, transported in exchange for protons within complex I of the electron transport chain.
Complex I transfers electrons, which come initially from our food, from the coenzyme NADH (nicotinamide adenine dinucleotide) to subsequent complexes in the chain. A part of this complex, however, performs an additional function as an exchanger of sodium ions for protons.
“This study brings two important contributions: the identification of a second fundamental function of complex I and the demonstration of the role of sodium in maintaining the membrane potential in mitochondria,” said Enríquez, in an interview with the RIDC Redoxoma website.
Since mitochondria lack a specific sodium transporter, the presence of a sodium-to-proton antiporter in complex I allows the membrane charge to remain stable. And whenever a proton gradient needs to be recovered, the same complex I can transfer the sodium gradient back into a proton gradient. “You can think of it as an energy store, like lithium or sodium batteries,” Enríquez said.
He highlighted the functional relevance of this discovery: “We provide a second fundamental function to a very old, very well-known protein. But the more relevant consequence is functionality. Because one thing that we all consider, since Peter Mitchell developed the chemiosmotic theory, is that the driver of the membrane potential in mitochondria is actually the electron transport chain that pumps protons against gradient, establishing a difference in proton concentration. But because protons are charged, you establish two things: A different concentration of protons, which means different pH, and a different charge, because it’s positively charged. Then, in principle, this defines what we call the membrane potential. But actually, the concentration of protons across the inner membrane doesn’t justify 100% the difference in membrane potential.”
According to Kowaltowski and Abdulkader, although the discovery is unexpected due to the low sodium concentration inside cells, Enríquez’s paper presents compelling data. The researchers used several experimental models, including mutants of respiratory chain components, as well as several methodological approaches, such as the use of different ionophores and sodium-depleted media. The experiments involved careful and quantitative bioenergetic measurements, including rare calibrated quantifications of membrane potential.
“They did quantitative bioenergetics, which is super rare in the literature. There are a lot of studies measuring membrane potential, but without calibrating it, without being quantitative,” said Kowaltowski. Recently, she published the video When and How to Measure Mitochondrial Inner Membrane Potentials, as well as a review on the subject.
The study also reveals that a point mutation in complex I associated with Leber hereditary optic neuropathy (LHON) specifically impairs the exchange of sodium for protons, without affecting electron transport or proton pumping through the complex. Leber hereditary optic neuropathy is a neurodegenerative mitochondrial disease affecting the optic nerve and is often characterized by vision loss in young adults.
For Kowaltowski, “the researchers not only describe a new mechanism central to the core of energy metabolism but also directly relate it to a disease.“
The article “Textbook oxidative phosphorylation needs to be rewritten”, de Alicia J. Kowaltowski and Fernando Abdulkader can be accessed here.
The article “A transmitochondrial sodium gradient controls membrane potential in mammalian mitochondria” is available here.
