Scientists have developed a new strategy to monitor a redox protein trafficking in cells
Protein disulfide isomerase (PDIA1) is an endoplasmic reticulum-resident enzyme whose classic function is to catalyze the insertion of disulfide bridges in nascent proteins so that they fold into the correct shape. However, PDIA1 is also found in the extracellular space, where it regulates events related to the internalization of viruses, thrombosis, platelet activation, and vascular remodeling. For years, scientists have been investigating how proteins from the PDIA1 family leave the endoplasmic reticulum and reach the extracellular space and specific pathways have not yet been clearly identified.
Now, researchers from the RIDC Redoxoma led by Francisco Rafael Martins Laurindo from the Instituto do Coração da Faculdade de Medicina at Universidade de São Paulo (USP), in collaboration with Professor Roberto Sitia from the Università Vita-Salute San Raffaele, in Milan, took a relevant step in this direction by developing a strategy to monitor the PDIA1 trafficking in cells based on the generation of an N-glycosylated variant of the protein, called Glyco-PDIA1. With this tool, they showed that a fraction of the PDIA1 pool leaves the reticulum and reaches the extracellular space through an unconventional route, bypassing the Golgi apparatus.
“This work opens up opportunities to develop approaches regarding strategies to inhibit extracellular PDIA1 because once we understand how the protein leaves the cell, there is the possibility of modifying or inhibiting this exit,” said postdoctoral researcher Percillia Victoria Santos Oliveira, first author of the article published in the Journal of Biological Chemistry (JBC). The researcher conducted part of the study with a FAPESP Research Internship Abroad (BEPE) grant in the laboratory of Professor Roberto Sitia, corresponding co-author of the article, considered one of the world leaders in subcellular protein trafficking.
According to Francisco Laurindo, “From a broader mechanistic perspective, this work provides a basis for a model of how the endoplasmic reticulum interacts with the extracellular environment. This interaction appears to be an important mechanism, given the functional effects associated with the presence of reticulum proteins on the cell surface. Interestingly, from a redox point of view, the reticulum is much more similar to the extracellular environment than the cytosol. The cytosol is highly reducing, and the reticulum is one of the most oxidizing compartments of the cell, with the extracellular environment being even more oxidizing than the reticulum.” For the researcher, the work provides a store of knowledge for applications that will impact in the medium and long term. “Without this type of basic knowledge, we are not feeding the system that will bring translational implications in the future.“
Strategy
Proteins synthesized in the rough endoplasmic reticulum are folded and directed to specific locations in cells, such as lysosomes and the plasma membrane, or secreted into the extracellular space. For this to happen, they need to be modified. In the reticulum, they can receive the addition of sugars, that is, be glycosylated, before proceeding to the Golgi complex, where they also receive other more complex sugar groups, which determine their final destination. They are then packaged again into vesicles, which migrate to their targets.
PDIA1, a protein with diverse cellular effects studied by Laurindo’s group, is one of the proteins resident in the reticulum through two mechanisms: first, it has a signal peptide that causes it to be translated into the reticulum; second, it has at its C-terminal end a KDEL (lysine-aspartate-glutamate-leucine) retrieval sequence. The KDEL amino acid sequence is recognized by KDEL receptors at the first station of the Golgi. Thus, when proteins tagged with this sequence leave the reticulum for the Golgi, they are sent back. This is a universal mechanism for proteins that are retrieved into the reticulum.
However, PDIA1 is also found in the extracellular space. According to the researchers, two main mechanisms can explain how the proteins escape the surveillance of the three KDEL receptors that operate in the Golgi: specific active secretion by unknown mechanisms or passive release by damaged or dead cells, from which the proteins escape due to the loss of plasma membrane integrity.
One of the objectives of the strategy developed to track PDIA1, according to Oliveira, was to show that it leaves the reticulum by an active mechanism and not by nonspecific release.
“Usually, proteins secreted from the cell or on the cell surface are glycosylated. They have a canonical glycosylation motif, which helps to direct them outside the cell. PDIA1 does not have this motif. So we imagined that, if we artificially inserted a glycosylation site, we could know where it went in the cell by looking at what type of modification these sugars undergo,” explained the researcher. The addition of the glycosylation motif did not disturb PDI’s function.
Proteins that pass through the Golgi acquire complex sugars. In HeLa cells expressing Glyco-PDIA1, the researchers initially observed that the protein had only immature sugars and, therefore, did not follow the classical secretion route, being retrieved to the ER.
They then manipulated the retrieval system. First, they removed the KDEL sequence, which caused the proteins to pass through the Golgi and be secreted. In this case, they found PDIA1 with complex sugars, confirming the escape of the recovery system back to the endoplasmic reticulum and its complete passage through the Golgi. Then, they decreased the expression of the KDEL receptors in the cells. To their surprise, they observed two pools of PDIA1, a fraction that passed through the Golgi and a fraction that did not. “We classified a static pool, which is larger and remains retained in the reticulum, and a mobile pool, which cycles between the reticulum and the Golgi. The latter would be the pool that escapes when we silence the receptors. This means that, in some way, the KDEL receptor is important for this alternative route of secretion from the reticulum to outside the cell,” explained the researcher.
Another important result of the study, according to Laurindo, was to present the first robust evidence that PDIA1 can gain access to the cytosol through a pathway dependent on the DNAJB12 chaperone, a protein in the reticulum membrane. Previous studies coordinated by Oliveira have already shown that DNAJB12 facilitates the cytosolic reflux of ER proteins during ER stress. Now, it has also been shown that this pathway may be independent of ER stress. In the cytosol, PDIA1 participates in several redox signaling pathways, such as the interaction with the NADPH oxidase (Nox) complex and the cellular cytoskeleton.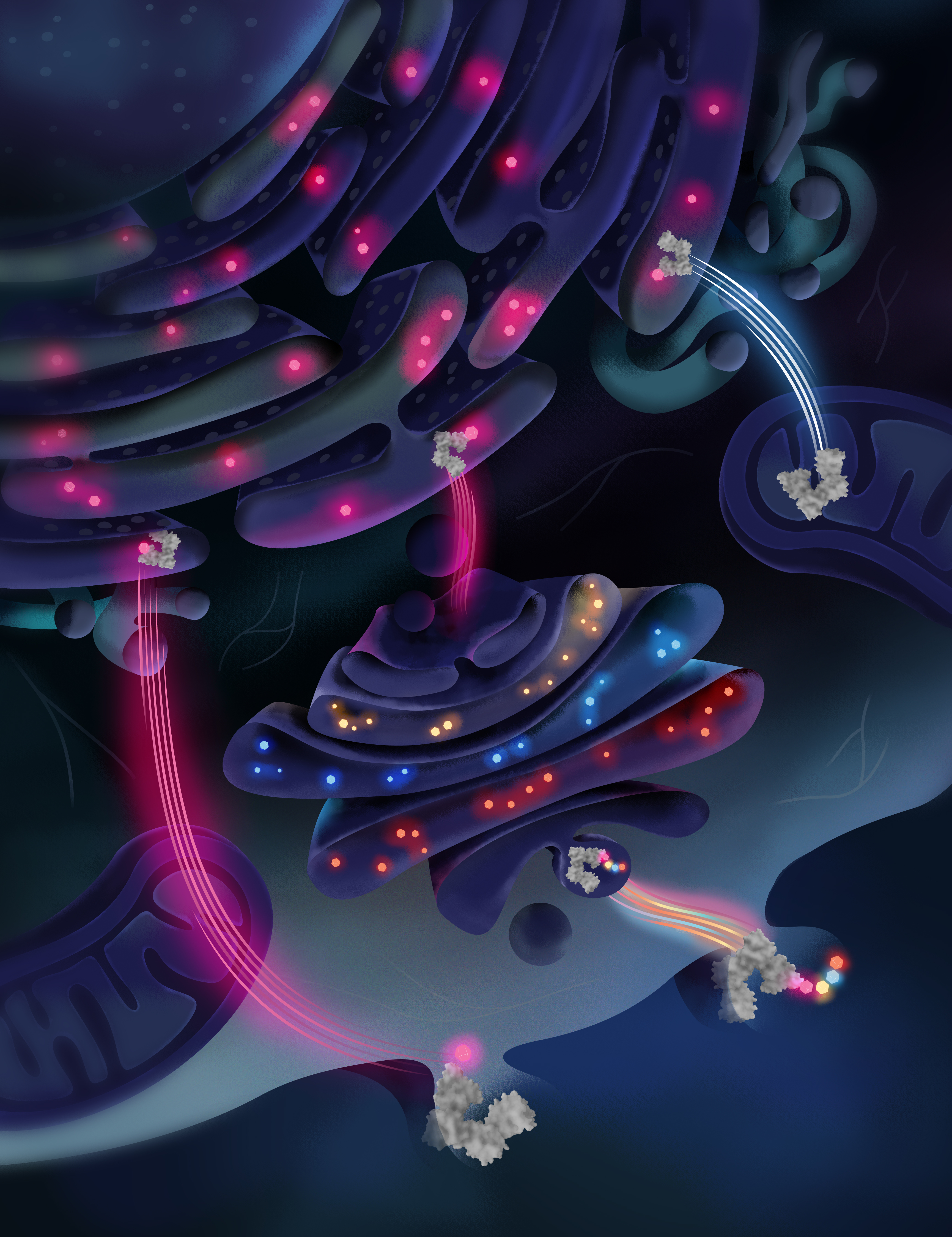
According to the researchers, the strategy developed in this study can be extended to investigate how other ER resident proteins reach the extracellular space.
The article Transport of Protein Disulfide Isomerase from the Endoplasmic Reticulum to the Extracellular Space without Passage through the Golgi Complex, de Percillia Victoria Santos Oliveira, Marco Dalla Torre, Victor Debbas, Andrea Orsi, Francisco Rafael Martins Laurindo and Roberto Sitia can be accessed here.
The article DNAJB12 and DNJB14 are non-redundant Hsp40 redox chaperones involved in endoplasmic reticulum protein reflux, by Aline Dias da Purificação, Victor Debbas, Leonardo Tanaka, Gabriele VM Gabriel, João Wosniak Júnior, Tiphany C De Bessa, Sheila Garcia-Rosa, Francisco RM Laurindo and Percillia VS Oliveira can be accessed here.
