New method detects a CO2-derived biological oxidant in cells
Researchers from the RIDC Redoxoma led by Professor Ohara Augusto from the Instituto de Química at Universidade de São Paulo (USP) revealed a new method for detecting peroxymonocarbonate in cells based on the use of fluorescent molecular probes. Peroxymonocarbonate is a biological oxidant produced by the reaction between hydrogen peroxide (H2O2 or hydrogen peroxide) and carbon dioxide (CO2). It is the first time that this oxidant has been detected in cells.
“Currently, evidence is accumulating that peroxymonocarbonate is important both in the adaptive responses of cells, that is redox signaling, and in cellular dysfunction. On the other hand, there is also epidemiological evidence that the CO2 levels we are close to reaching in urban contemporary societies cause physiological problems. And CO2 toxicity mechanisms are still poorly understood. So this work is important not only for providing a method to show that peroxymonocarbonate is produced in a range of conditions, including in cels but also for discussing it, considering the limited attention that CO2 has received in the redox area,” Augusto said.
The results of the work, which has the collaboration of Uruguayan researchers Rafael Radi and Natalia Rios, were published in the journal Chemical Research in Toxicology. “I considered it appropriate to submit to this journal because we published there our first works on the involvement of peroxymonocarbonate in biological processes when the oxidant was ignored in biology,” the researcher said.
Method
To detect peroxymonocarbonate, the researchers used fluorescence measurements with boronate probes. First, they generated physiological concentrations of hydrogen peroxide in an enzymatic reaction at steady-state, that is, at constant production, and measured the fluorescence of a boronate probe in the presence and absence of CO2. Boronates detect oxidants such as hydrogen peroxide, peroxynitrite, hypochlorous acid, and peroxymonocarbonate, which react with them at different rates and intensities, allowing the identification of these oxidants. In 2018, Augusto and Truzzi demonstrated that peroxymonocarbonate oxidizes boronates faster than hydrogen peroxide.
The cell study was carried out with macrophages activated to generate hydrogen peroxide. Macrophages are immune system cells that, depending on the type of activation, generate different oxidants. The researchers conducted several controls to conclude that the cells generated neither peroxynitrite nor hypochlorous acid but rather peroxymonocarbonate when in the presence of CO2.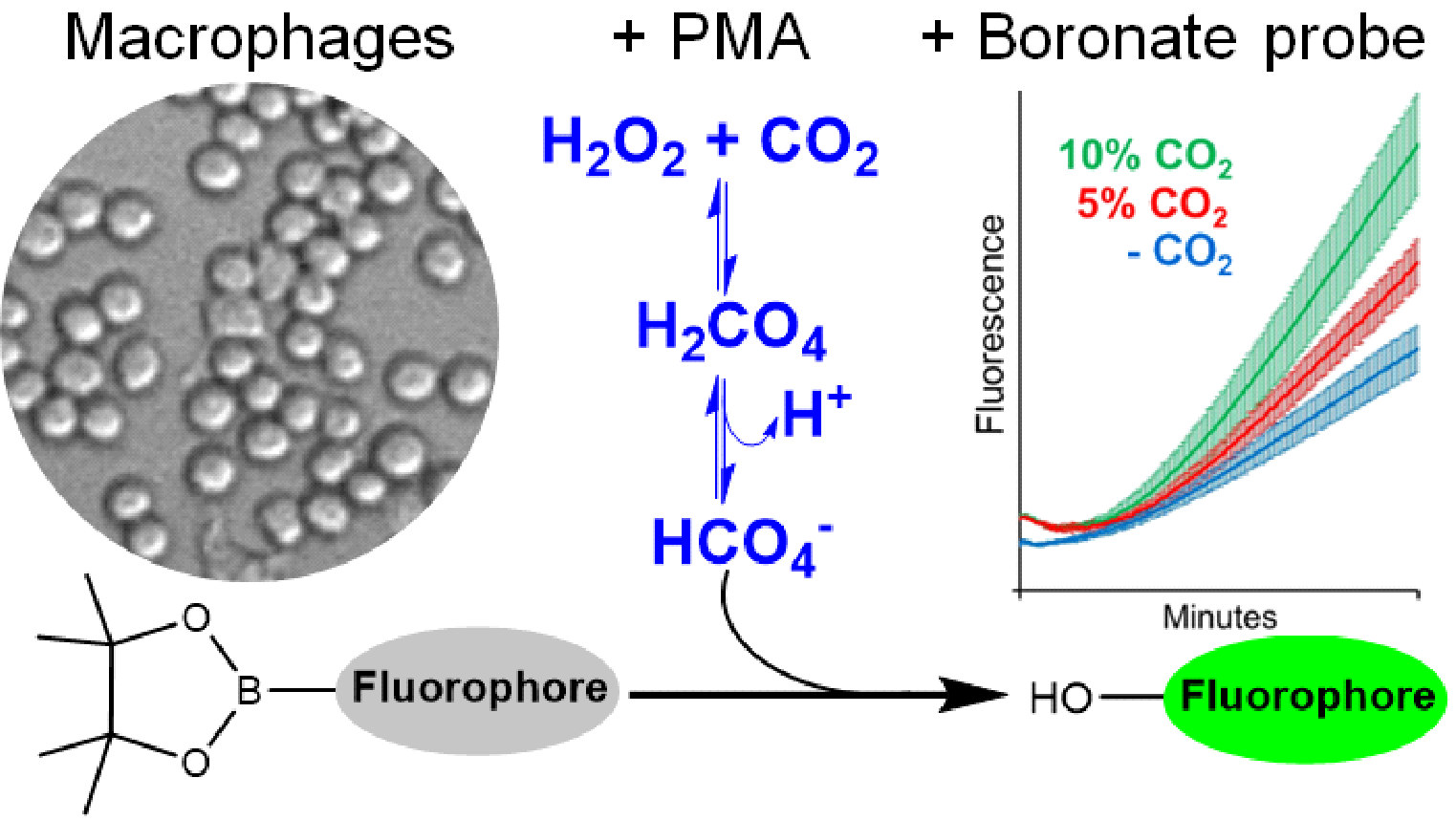
“This is a relatively simple method for detecting peroxymonocarbonate at physiological concentrations of hydrogen peroxide and CO2. Previously it was impossible, but today, researchers may consider that some effects they observe in cells, such as higher oxidation of certain proteins or cell responses, could be due to peroxymonocarbonate and they will be able to test this,” the researcher said.
Despite being an oxidant known to chemists since the 1960s and having technological applications as a disinfectant and whitener, peroxymonocarbonate formation in cells was considered practically impossible due to the low concentrations of its precursors and its formation rate. Augusto says that only in the 2000s did the oxidant begin to be investigated in biological systems and, initially, the focus was on oxidative damage.
In 2006, Augusto and her group published an article in the Chemical Research in Toxicology (CRT) showing that peroxymonocarbonate accelerated the hydrogen peroxide-mediated oxidation of glutathione and albumin and suggested it could be a biological oxidant. Furthermore, these results added to emerging data that the main physiological buffer, the CO2/bicarbonate buffer, was redox active.
In 2019, New Zealand researcher Christine Winterbourn and collaborators showed in cells that signaling mediated by the epidermal growth factor (EGF) depended on the presence of CO2 and proposed that it would be mediated by peroxymonocarbonate. In the same year, the groups of Augusto and Winterbourn independently demonstrated that peroxymonocarbonate promoted the superoxidation of 2 Cys peroxiredoxins, enzymes also involved in redox signaling.
Since then, research on peroxymonocarbonate and the effects of CO2 on aerobic organisms has attracted more attention in the literature.
Redox signaling and CO2
Redox signaling is an adaptive response. “There are various levels of stress. When there is a slight increase in stress, the cell adapts. The formation of oxidants can, for example, lead to the expression of genes for antioxidant enzymes to respond, in this case, to oxidative stress. And many pathways that lead to cellular responses involve thiol proteins, which peroxymonocarbonate oxidizes faster than hydrogen peroxide”, explains Augusto, adding that irreversible cellular damage only occurs with a large formation of oxidants.
CO2 is one of peroxymonocarbonate precursors, along with hydrogen peroxide. The gas is naturally present in the atmosphere and is a normal constituent of the human body, which exhales about 1.0 kg of CO2 per day as a metabolism product.
From a redox point of view, CO2 modulates the reactivity of hydrogen peroxide and peroxynitrite, two important metabolites of molecular oxygen. Furthermore, it alters the expression of genes, including those involved in inflammation. It also is involved in protein nitration via peroxynitrite and in protein carbamylation, another post-translational modification that can alter the biological function of proteins. Although more evidence of its role as a biological oxidant is needed, peroxymonocarbonate appears as one of the possible intermediaries of the harmful effects of increased levels of CO2 in our bodies. The researcher highlights that CO2 also acts through non-redox mechanisms.
In a review article published in the journal Nature Sustainability in 2019, American researchers warned of the potential health risks of exposure to elevated levels of ambient CO2, including inflammation, reductions in higher-level cognitive abilities, bone demineralization, kidney calcification, oxidative stress, and endothelial dysfunction. According to the authors, it was traditionally considered that CO2 levels would need to reach a concentration of at least 5,000 parts per million (ppm) before affecting human health. However, research suggests that CO2 levels as low as 1,000 ppm can cause health problems, even if exposure lasts just a few hours.
The article “Production of Peroxymonocarbonate by Steady-State Micromolar H2O2 and Activated Macrophages in the Presence of CO2/HCO3− Evidenced by Boronate Probes”, by Edlaine Linares, Divinomar Severino, Daniela R. Truzzi, Natalia Rios, Rafael Radi, and Ohara Augusto can be accessed here.
