Neutrophils adopt different defense strategies depending on the stimulus
Neutrophils serve as the body’s first line of defense against pathogens like viruses, bacteria, and fungi. They also play a significant role in regulating inflammation. To neutralize threats, they employ various mechanisms, including degranulation, phagocytosis, and the release of extracellular traps (NETs), structures formed by DNA and microbicidal proteins.
In a new study, researchers from RIDC Redoxoma, led by Professor Graziella Eliza Ronsein from the Instituto de Química at the Universidade de São Paulo (USP), investigated how neutrophils respond to two well-known activators - PMA and ionomycin - and discovered that activation occurs through distinct biochemical pathways. The results show that these cells modulate their immune response according to the stimulus received.
“In the literature, all stimuli that neutrophils receive are treated the same way, as if the entire neutrophil response was the same. Our study shows that neutrophils react differently depending on the stimulus, and the consequences of these responses appear to be quite different as well,” explains Ronsein.
These different responses may have important implications for understanding inflammatory and autoimmune diseases. “We observed that the citrullinated peptides generated by the activation of neutrophils with ionomycin are very similar to those formed when neutrophils interact with certain specific bacterial toxins. And many of these peptides are found to act as autoantigens involved in autoimmune diseases, such as rheumatoid arthritis,” said PhD student Rafaela Oliveira Nascimento, co-first author of the article published in Redox Biology.
Defense strategies
A well-known strategy of the immune response involves rapidly producing large amounts of reactive species. During the course of an infection, neutrophils are recruited to the affected site and activate the enzyme NADPH oxidase, which generates the superoxide radical. This radical serves as a precursor to hydrogen peroxide and other reactive species formed by myeloperoxidase.
However, the researchers found that only PMA-activated neutrophils generate reactive species, while those stimulated by ionomycin do not produce these molecules.
Degranulation, another key defense mechanism, involves the release of granules containing cytotoxic enzymes. Proteomic analysis revealed that while PMA triggers mild degranulation, ionomycin induces massive degranulation of primary and secondary granules.
In an earlier study, the research team developed a method to isolate neutrophil granules from small blood samples and provided the first proteomic characterization of these organelles.
Another crucial antimicrobial strategy is the formation of neutrophil extracellular traps (NETs). As neutrophils release NETs, they undergo a specialized form of cell death known as NETosis, triggered by various stimuli.
The study showed that both PMA and ionomycin induce NET formation but with significant differences. Live-cell microscopy revealed that ionomycin-triggered NET formation occurs much faster than that induced by PMA, indicating distinct underlying mechanisms.
Citrullination
The research also revealed an extensive citrullination process in proteins from ionomycin-activated neutrophils, including essential cytoskeleton, nucleus, and NADPH oxidase components. According to Nascimento, citrullination of NADPH oxidase components could explain the enzyme inactivation and the absence of superoxide production in ionomycin-activated neutrophils.
Ionomycin increases intracellular calcium levels, leading to the activation of an enzyme called PAD4, which converts arginine residues into citrulline. This protein modification is called citrullination.
In histones, proteins that organize DNA, arginine’s positive charge interacts with DNA’s negative phosphate groups, maintaining chromatin structure. When citrullinated, histones lose their positive charge, leading to chromatin decondensation and facilitating NET formation through a mechanism independent of reactive species.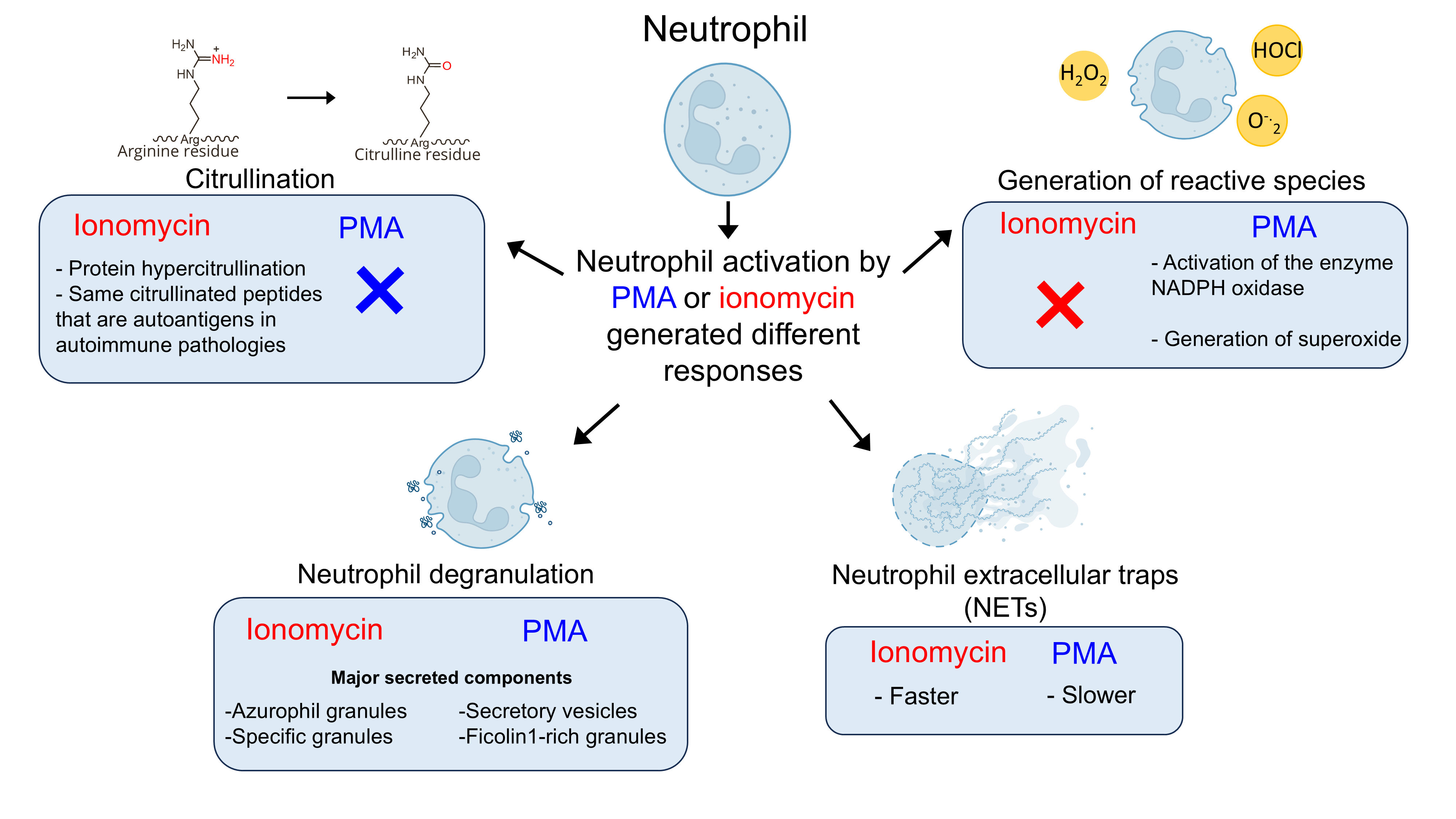
Many citrullinated proteins detected in this study have previously been identified as autoantigens in autoimmune diseases. Autoantigens are molecules that cause an immune response against the body’s own tissues. The uncontrolled release of NETs is considered a source of autoantigens and is associated with autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus. According to the researcher, this mechanism of neutrophil response independent of NADPH oxidase activation may play a role in autoimmune diseases.
In a prior study, Ronsein’s team mapped citrullinated proteins in ionomycin-activated neutrophils, identifying changes that precede NET formation. Proteomic data obtained by the group is publicly available.
As a next step, the researchers plan to investigate how neutrophils respond to milder stimuli, such as physiological ones, providing further insights into immune regulation. “Ionomycin is a very strong stimulus that rapidly remodels intracellular proteins,” says Ronsein.
The article Investigating Neutrophil Responses to Stimuli: Comparative Analysis of Reactive Species-dependent and Independent Mechanisms, by Lorenna Rocha Reis, Rafaela Oliveira Nascimento, Mariana Pereira Massafera, Paolo Di Mascio, and Graziella Eliza Ronsein, can be accessed here.
