How hyperglycemia increases the risk of thrombosis
Research by scientists from the RIDC Redoxoma has revealed new mechanisms by which hyperglycemia causes thrombosis. The study may lead to targeted strategies to prevent cardiovascular dysfunction in people with diabetes or those who have prolonged hyperglycemia. The results were published in the Journal of Thrombosis and Haemostasis.
“The main cause of death in the Brazilian population and several other Latin American countries are ischemic events, cardiovascular diseases, such as heart attack and ischemic stroke, in which arterial thrombosis is the precipitating causal event. These diseases can be precipitated by a series of risk factors, such as hyperglycemia, dyslipidemia, and hypertension. Of these factors, hyperglycemia appears to be very significantly associated with cardiovascular diseases. So, the relevance of the problem we are addressing starts from there,” said Renato S. Gaspar, who conducted the research during his postdoctoral studies under the supervision of Francisco Rafael Martins Laurindo from the Instituto do Coração da Faculdade de Medicina at Universidade de São Paulo (USP) and a member of the RIDC Redoxoma. Gaspar is currently a professor at the Departamento de Medicina Translacional at Universidade Estadual de Campinas (Unicamp).
Hyperglycemia occurs when there is excess glucose in the blood and is a manifestation of diabetes. In a study published in 2022 in the journal PLOS ONE, the researchers showed that, in Brazil, between 2005 and 2017, hyperglycemia was the risk factor most associated with deaths from cardiovascular diseases compared to other modifiable factors.
Conditions of prolonged hyperglycemia, such as diabetic ketoacidosis, are associated with an increased risk of thrombosis by causing endothelial dysfunction, which promotes platelet adhesion and the formation of thrombi or blood clots. The endothelium is the inner lining of blood vessels, and platelets are cells produced by the bone marrow that circulate in the bloodstream and help the blood clot.
Now, researchers have shown that, in hyperglycemia, endothelial peri/epicellular protein disulfide isomerase A1 (pecPDI) regulates the interaction between platelets and the endothelium through adhesion-related proteins and changes in the biophysics of the endothelial membrane.
“We have shown a PDI pathway in arterial cells that mediates thrombosis in diabetes under hyperglycemia conditions, involving a specific molecular mechanism, which has been identified,” Francisco Laurindo said.
PDI is an endoplasmic reticulum enzyme that has the classic function of catalyzing the insertion of disulfide bonds into nascent proteins so that they fold into the correct shape. It is also found in the extracellular medium as a secreted pool or bound to the cell surface, the pecPDI, in several cell types, including platelets and endothelial cells. Studies have shown that pecPDI regulates thrombosis in several models.
Biochemical and biophysical modifications
To investigate the relationship between platelets and the endothelium in hyperglycemia, the researchers created a model with human umbilical vein endothelial cells (HUVECs) cultured in different glucose concentrations. In this way, they produced normoglycemic cells with normal glucose levels and hyperglycemic cells with excess glucose. The contribution of protein disulfide isomerase A1 (PDI) was evaluated using whole-cell PDI or pecPDI inhibitors.
Initially, the cells were incubated with platelets collected from healthy individuals. Platelets adhered almost 3-fold more onto hyperglycemic cells compared with normoglycemic ones. Since PDI inhibition abolished this effect, the researchers concluded that the process is regulated by endothelial pec-PDI.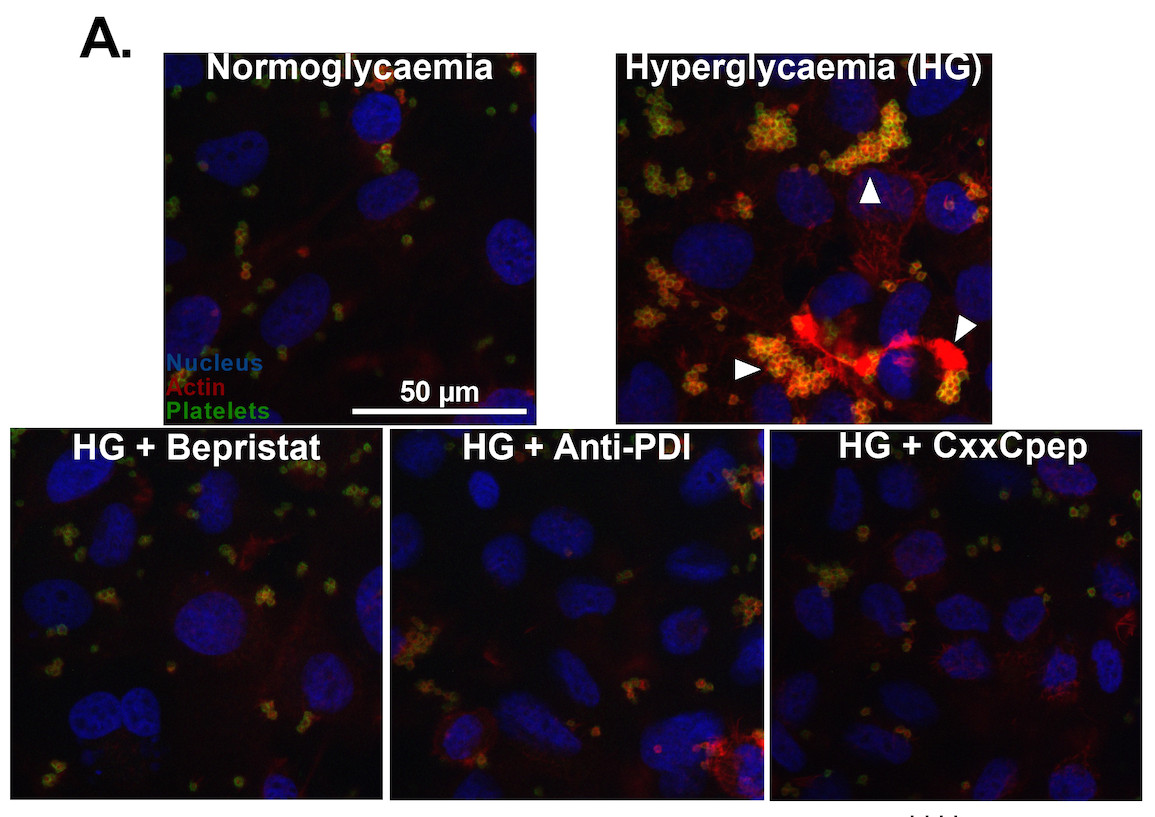
To better understand the results, they investigated biophysical processes, such as remodeling endothelial cell cytoskeleton. They found that hyperglycemic cells had better-structured actin filament fibers than non-hyperglycemic cells. They also measured hydrogen peroxide production since reactive oxygen species mediate cytoskeletal reorganization and cell adhesion. In this case, hyperglycemic cells generated twice as much hydrogen peroxide as normoglycemic cells.
The next step was to investigate whether the reorganization of the cytoskeleton affected cell membrane stiffness since platelets tend to adhere to more rigid surfaces. Using atomic force microscopy, they proved hyperglycemic cells were more rigid than normoglycemic cells. The atomic force microscopy studies were conducted with researcher Luciana Magalhães Rebelo Alencar’s group at the Departamento de Física at Universidade Federal do Maranhão.
For Gaspar, it was important to address biochemical and biophysical aspects in the work. “I think we rarely think about Biophysics, for example, the stiffness of proteins. Showing that PDI somehow regulates this stiffness in the endothelial cell may open up a way of thinking beyond Biochemistry.“
The images produced by microscopy also showed the formation of cell protrusions, with extracellular vesicles that seemed to be detached from the protrusions. This observation led the researchers to investigate the set of proteins secreted by the cells, the secretome, to see if they were releasing proteins that could increase platelet adhesion. “The idea of this experiment was to detect proteins that would be exclusively expressed or present in hyperglycemic cells and not in control cells or cells treated with PDI inhibitors,” Gaspar explained. To do this, they conducted proteomic studies in collaboration with the group of researcher Graziella Eliza Ronsein from the Instituto de Química at USP and a member of the RIDC Redoxoma.
In the secretome, they found 947 proteins, of which they selected eight with a role in cell adhesion. They then reduced the gene expression of three of these proteins using the RNA interference tool and arrived at two proteins, SLC3A2 and LAMC1, as modulators of platelet adhesion. SLC3A2 is a membrane-bound protein, and LAMC1 is the gamma subunit of laminin, a key extracellular matrix component.
The conclusion was that exposure to hyperglycemia induced the secretion of specific adhesion-related proteins and that inhibition of PDI and pecPDI prevented endothelial cells from secreting these proteins.
Application
PDI is a protein involved in the formation of thrombi. In previous independent studies, Gaspar and Laurindo demonstrated the relationship between PDI and NADPH oxidase 1 (Nox 1) in different situations, including platelet adhesion. Noxs are enzymes that catalyze the reduction of molecular oxygen, generating the superoxide radical anion, which, in turn, participates in other oxidants generation.
According to the researchers, clinical trials with pec-PDI inhibitors are already underway, focusing on diseases such as sickle cell anemia and cancer-associated thrombosis. “Our work adds a perspective of treatment to a new disease, diabetes. Perhaps we can look at secondary prevention of ischemia in diabetes,” Gaspar said.
For Laurindo, the prospect that there may be specific thrombosis inhibitors in diabetes includes not only PDI but the protein SLC3A2 and LAMC1. “Inhibiting laminin is a little more complicated because it is a protein that has several functions, but SLC3A2 would be a potential target, and there is nothing in the literature about this protein inhibition as an antithrombotic.“
The article Endothelial protein disulfide isomerase A1 enhances membrane stiffness and platelet-endothelium interaction in hyperglycemia via SLC3A2 and LAMC1, by Renato S. Gaspar, Álefe Roger Silva França, Percillia Victoria Santos Oliveira, Joel Félix Silva Diniz-Filho, Livia Teixeira, Iuri Cordeiro Valadão, Victor Debbas, Clenilton Costa dos Santos, Mariana Pereira Massafera, Silvina Odete Bustos, Luciana Magalhães Rebelo Alencar, Graziella Eliza Ronsein, and Francisco R. M. Laurindo, can be accessed here.
