Adiponectin reverses pancreatic beta cell damage linked to obesity
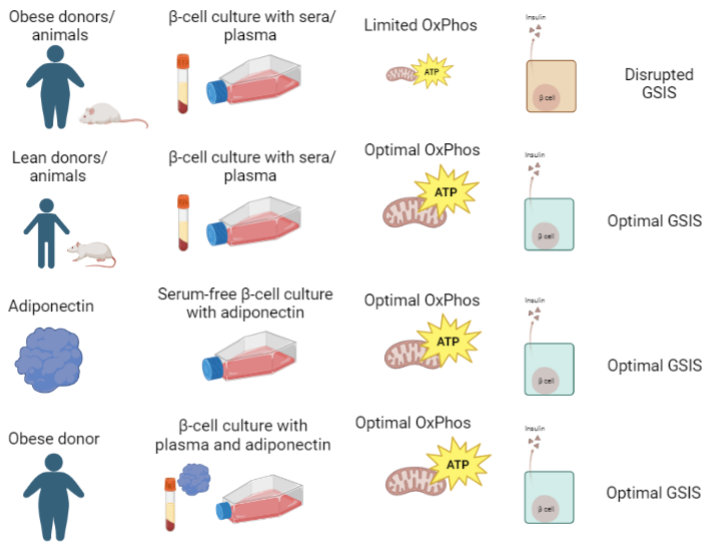
Scientists have shown for the first time that adiponectin, a hormone released by adipose tissues, can modulate glucose-stimulated insulin secretion and metabolic fluxes in pancreatic beta cells. The addition of adiponectin completely restores beta cells functional integrity compromised by treatment with plasma from obese donors, indicating that the lack of the hormone causes dysfunction. Beta cells are responsible for synthesizing and secreting insulin, and their dysfunction is related to type 2 diabetes.
“This work opens the door to new research focusing on the effects of adiponectin on beta cells, exploring mechanistic pathways, for example. This was an answer that we gave to our original research question, and that opened a million other questions to be explored in future studies”, said Ana Cláudia Munhoz, first author of the article, published in the journal Aging Cell. The research was carried out during her postdoctoral studies, under the supervision of Professor Alicia Kowaltowski, from the Instituto de Química at Universidade de São Paulo (USP) and a member of the RIDC Redoxoma.
Adiponectin is found at elevated levels in lean individuals. In the study, the researchers showed that incubating beta cells with serum or plasma from obese rats and humans hampers mitochondrial oxidative phosphorylation and glucose-stimulated insulin secretion (GSIS) relative to serum and plasma from lean rats and humans. They also showed that adiponectin alone, in the absence of serum, supports beta cell function and reverses the harmful effects of obese plasma.
For Kowaltowski, these results indicate a new, specific, and highly effective way to protect the beta cells and prevent their degeneration related to obesity. “This means that we have good potential to design drugs in the future that prevent pancreatic degeneration in obesity. The effect of adiponectin in this model is so large and it is so reproducible in different animal models that it brings me a lot of hope.”
About half a billion people are currently living with type 2 diabetes mellitus, and this number is expected to increase by 51% until 2045 as obesity rates continue to rise and the population ages. Type 2 diabetes is a chronic disease, related to age, characterized by uncontrolled glucose homeostasis in the blood, caused by a decrease of the effects of insulin (called insulin resistance), or by inadequate release of insulin by pancreatic beta cells, which, in turn, also hampers insulin signaling.
Adiponectin
The researchers already knew that serum from animals under caloric restriction diets had a protective effect on pancreatic islets, primary beta cells, and insulin-secreting cell lines in vitro, based on previous work by the group.
Obesity increases the incidence of age-related dysfunctions, including beta-cell dysregulation leading to inadequate insulin secretion. “Our lab has been interested for many years in mechanisms involved in protection from the effects of aging associated with being obese or not being obese. And calorie restriction is an animal model that prevents obesity. It is not a model of ultra-thinness”, emphasizes Kowaltowski.
They also discovered that incubation for just 24 hours with serum from moderately obese animals fed ad libitum (AL) led to an immediate worsening of beta cell function, impairing insulin secretion. “We knew that this occurred independently of nutrients, but we didn't know why, which molecular factors were involved”, says Munhoz. They had a clue, however: in vascular cells, the increase in mitochondrial electron transport capacity promoted by caloric restriction was associated with adiponectin-activated eNOS signaling.
Based on this data, the researchers investigated what happened to beta cells when incubated with plasma from lean and obese people and with serum from rats subjected to a 60% calorie-restricted diet compared to animals fed ad libitum (AL), which over time develop obesity, insulin resistance, and other characteristics of the metabolic syndrome.
Plasma samples from a group of lean and obese men and women without pathologies were obtained from the A.C. Camargo Cancer Center Biobank, created by researchers Vilma Martins and Tiago Goss. Interestingly, the plasma of women, who have higher levels of adiponectin, had better effects than the plasma of men. In addition, the researchers quantified adiponectin in the samples and also carried out experiments with serum from knockout mice unable to produce adiponectin, supplied by researcher Francielle Mosele, from Universidade Estadual Paulista (Unesp).
Thus, they proved that adiponectin present in the blood of lean rats and mice, as well as in the plasma of lean women, stimulates metabolic fluxes in beta cells. And, by adding the hormone to cells incubated with plasma from obese donors, they saw that adiponectin alone reverses the harmful effects of obese plasma on beta cells.
Adiponectin has been extensively studied in aging and metabolic syndrome, using both animal models and population observations. It acts on cells by binding to adiponectin receptors and modulating protein kinase B (AKT) phosphorylation. It also acts via the hypothalamus to inhibit appetite and increase energy expenditure, promoting fatty acid oxidation in muscle and liver, leading to weight loss. Overall, studies indicate that adiponectin is an important regulator of energy metabolism in various organs.
However, according to Kowaltowski, “There is still a lot to study, adiponectin is a mysterious hormone. Its levels increase with age, but apparently, we lose sensitivity to it, as with insulin and leptin. We still don't fully understand unresponsiveness to adiponectin, nor why women have higher levels of this hormone. There are also data showing that central adiposity decreases adiponectin, but the mechanisms related to its production and secretion are still not fully understood. The robust effect of this hormone on beta cells that we uncovered in this work demonstrates that we should invest in understanding its full effects better.”
The article Adiponectin Reverses 𝛽-Cell Damage and Impaired Insulin Secretion Induced by Obesity, by Ana Cláudia Munhoz, Julian D. C. Serna, Eloisa Aparecida Vilas-Boas, Camille C. Caldeira da Silva, Tiago Goss dos Santos, Francielle C. Mosele, Sergio L. Felisbino, Vilma Regina Martins and Alicia J. Kowaltowski, can be read here.
