A new mechanism of import and maturation of the main antioxidant in human mitochondria has been revealed
Mitochondrial energy metabolism is the main source of superoxide radicals and hydrogen peroxide (H2O2) in most eukaryotic cells. Under physiological conditions, H2O2 is continuously generated in a regulated manner, participating in processes such as cell differentiation and aging. However, in high concentrations, it can induce damage to proteins, membranes, and mitochondrial DNA, impairing ATP production and compromising vital metabolic functions. Thus, H2O2 plays a dual role in cell signaling and oxidative damage.
To understand the functions of H2O2, it is important to know in which compartments of the mitochondria this oxidant is generated and where it is degraded. Peroxiredoxin 3 (Prdx3) is the main enzyme responsible for removing H2O2. It is synthesized in the cytoplasm by ribosomes and then transported to the mitochondria. However, the mechanisms that regulate this transport were, until now, unknown.
Now, researchers from the RIDC Redoxoma have elucidated the molecular mechanisms of Prdx3 transport from the cytosol to the mitochondria. Furthermore, they revealed that Prdx3 is present not only in the mitochondrial matrix, as previously believed, but also in the intermembrane space.
Mitochondria have two membranes, an internal and an external membrane, which organize the organelle functionally and spatially. The space between the two membranes delimits the so-called intermembrane space, while the mitochondrial matrix is delimited by the internal membrane.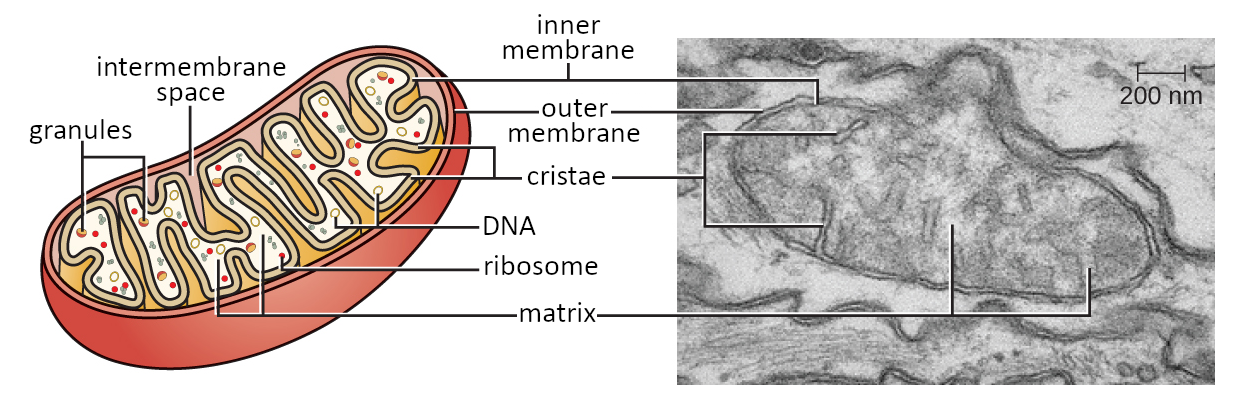
“We demonstrated that Prdx3 is located in two mitochondrial compartments, and this knowledge opens new perspectives in the study of its functions, with impacts on the understanding of mitochondrial H2O2-mediated cell signaling,” said researcher Fernando Gomes, lead author of the work published in the journal Redox Biology. Gomes is a postdoctoral fellow at the Laboratory of Proteins and Redox Biology at the Instituto de Biociências at Universidade de São Paulo (USP), under the supervision of Professor Luis E.S.Netto, a member of the RIDC Redoxoma.
According to the researchers, the dual localization of Prdx3 raises intriguing questions about its specific roles in each compartment. “In the matrix, I see more of an antioxidant role because a lot of H2O2 is generated there. In the intermembrane space, I believe that Prdx3 may have a signaling effect or even participate in the formation of disulfide in target proteins. However, we do not yet have evidence of this,” Netto explained.
Another important observation is that the intermembrane space acts as a barrier, preventing the diffusion of mitochondrial H2O2 into the cytosol. Prdx3 in this compartment likely plays a key role in controlling hydrogen peroxide diffusion.
Netto emphasizes that the volume of the intermembrane space is much smaller than that of the matrix and, in general, much less studied. “Perhaps the discovery that Prdx3 is also located in the intermembrane space will help reveal other processes occurring in this mitochondrial compartment.”
Mechanism
The researchers determined Prdx3’s dual localization and investigated the enzyme’s import mechanisms using highly purified mitochondria from HEK293T cells.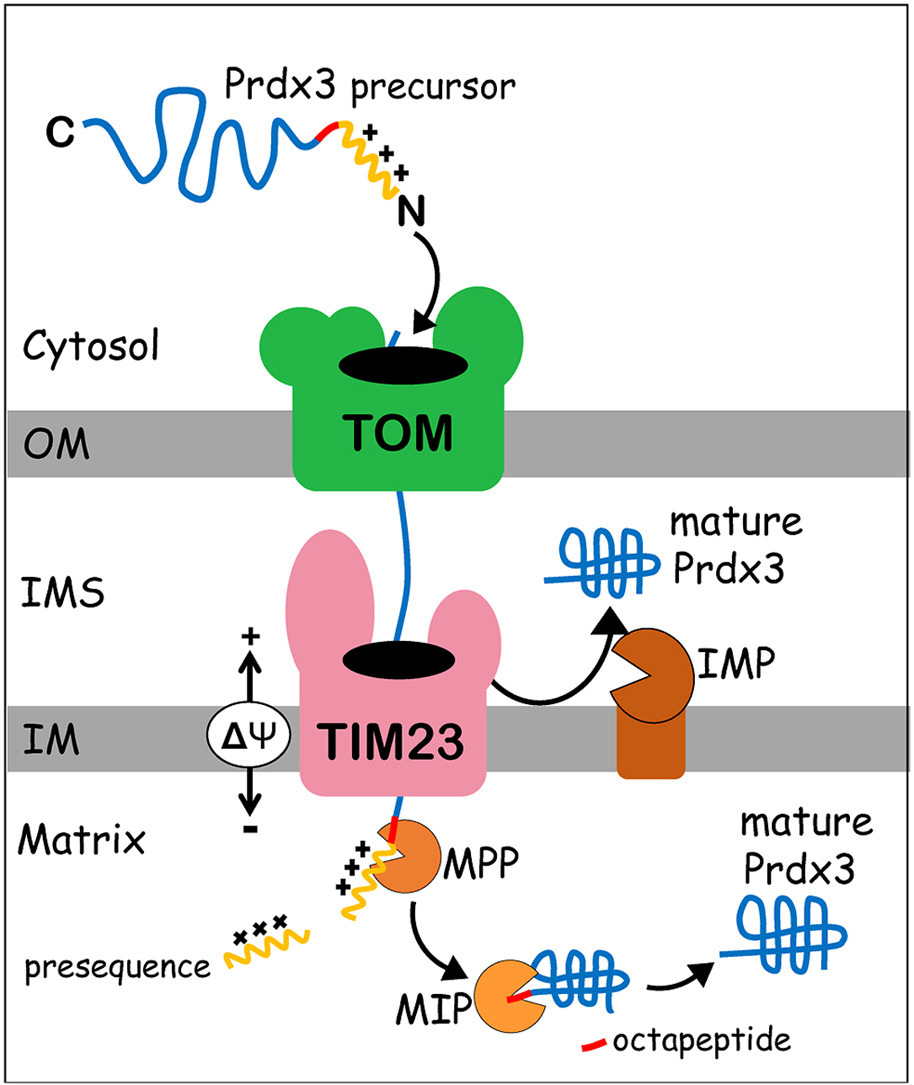
Most mitochondrial proteins (99%) are encoded by nuclear genes, translated into the cytoplasm, and subsequently imported into the organelle by protein transport complexes in the outer and inner membranes. Many of these proteins are synthesized with a specific sequence of amino acids located in the N-terminal region called the presequence, a targeting signal for the mitochondrial matrix. After the import, most of the presequences are removed by the action of mitochondrial processing peptidase (MPP), resulting in mature mitochondrial proteins. However, a subset of proteins undergoes additional processing steps, for example, by the action of mitochondrial intermediate peptidase (MIP).
Import of Prdx3 into the mitochondrial matrix follows this process. “The protein directed to the matrix is first cleaved by the MPP protease, which removes the presequence. After this first cleavage, MIP performs a second cleavage to remove eight additional amino acids. We have long wondered what the cause of this second cleavage is since the first removes a large part of the signal. We believe that the removal of these eight amino acids is important to make Prdx3 more stable in the organelle,” said Gomes.
Using previously published mitochondrial proteomics data, the researchers created a comprehensive list of human, mouse, and rat proteins containing the R-10 motif, which is part of the presequence of proteins cleaved by both MPP and MIP. Through these analyses, they determined for the first time a consensus site for sequential cleavage by these proteases. They also showed that all components of the mammalian mitochondrial thioredoxin/peroxiredoxin antioxidant system possess the R-10 motif, indicating that they also likely undergo sequential processing by MPP and MIP during their maturation.
By expressing human Prdx3 in a wild-type yeast strain and null mutants for another mitochondrial protease, the researchers showed that the protein’s targeting to the intermembrane space relies on the inner membrane peptidase (IMP) complex. However, as Netto points out, there are still unresolved questions. “The same amino acid sequence directs the protein to two different locations. Understanding this remains a challenge for us.”
Perspectives
Prdx3 has a complex catalytic cycle. Interestingly, when there is excess hydrogen peroxide, the enzyme can become hyperoxidized, losing its ability to eliminate hydrogen peroxide, which can then act on redox signaling. Furthermore, “When reduced, it forms a 12-unit oligomer; when oxidized, it adopts a dimeric form. It is even curious from a biochemical point of view as it transitions from a dimer to a dodecamer”, said Netto.
To study the physiological significance of Prdx3’s dual localization in mitochondria, Gomes created a cell line lacking the enzyme using CRISPR-Cas9 technology. Now, he plans to introduce a plasmid expressing the protein targeted exclusively to the matrix or intermembrane space to analyze H2O2 diffusion dynamics.
Recent research highlights the broader relevance of Prdx3 in both physiological and pathological processes. “One of these studies showed the involvement of hyperoxidated Prdx3 in signaling ferroptosis, a type of unprogrammed cell death. Another study showed the importance of Prdx3 in the maintenance and self-renewal of cancer stem cells, specifically in glioblastoma, a type of brain tumor. Studying the role of the dual mitochondrial localization of Prdx3 in these processes will be fundamental,” Gomes remarked.
The article Human mitochondrial peroxiredoxin Prdx3 is dually localized in the intermembrane space and matrix subcompartments by Fernando Gomes, Helena Turano, Luciana A. Haddad, and Luis E.S. Netto can be accessed here.
