Study shows how mitochondria regulate autophagy via calcium signaling
In an article published in The FASEB Journal, researchers from RIDC Redoxoma led by professor Alicia Kowaltowski from the Instituto de Química at USP demonstrated that the NCLX transporter, responsible for mitochondrial calcium efflux, is an important regulatory node that integrates mitochondria, control of autophagy by calcium ions and cellular responses to nutrient availability. Thus, they established the link between autophagy and mitochondrial calcium, two fundamental processes in energy metabolism regulation.
“This work is important because we linked these processes. Since both mitochondria and autophagy are involved in metabolism, it makes sense that there is coordination between them. Normally when we study cell biology, things are seen separately, and we need to remember that, in cells, one process depends on the other and regulates the other. Although this work is basic science, which seems somewhat abstract, this knowledge can certainly be used in pathological contexts and to develop therapeutic targets,” said Vitor de Miranda Ramos, who proposed investigating the link between these processes during his doctorate, and is the first author of the article.
In addition to generating energy for cells, mitochondria directly participate in several calcium-sensitive cellular regulatory pathways, as they can take up, absorb and release calcium ions. Mitochondrial uptake of calcium ions is mediated by the mitochondrial calcium uniporter complex (MCU), while the mitochondrial Na+/Li+/Ca2+ exchanger (NCLX) moves calcium ions from the mitochondrial matrix to the intermembrane space in exchange for sodium ions.
Calcium affects almost every aspect of cellular life. Calcium ions are known second messengers in metabolic signaling and play an important role in autophagy regulation.
Autophagy
Autophagy is the evolutionarily-conserved degradation and recycling of cellular components, with a basal role in maintaining cellular homeostasis. Due to its ability to remove unwanted elements and promote nutrient availability, autophagy is necessary for quality control, tissue renewal, and metabolic regulation.
In addition to its basal function, autophagy is also activated in response to decreased nutrient availability and is considered one of the mechanisms responsible for the benefits of calorie restriction. In previous work with animals on caloric restriction, Kowaltowski’s group had already found that variations in diet affected mitochondrial calcium transporters.
In this work, as a starting point to understand the interaction between mitochondrial calcium transport and autophagy, the researchers subjected mice to four months of caloric restriction and observed that mitochondria isolated from the livers of these animals contained higher levels of NCLX than those from animals submitted to an ad libitum diet.
From this, using liver cells in culture, they created models to mimic calorie restriction and again observed increased NCLX expression.
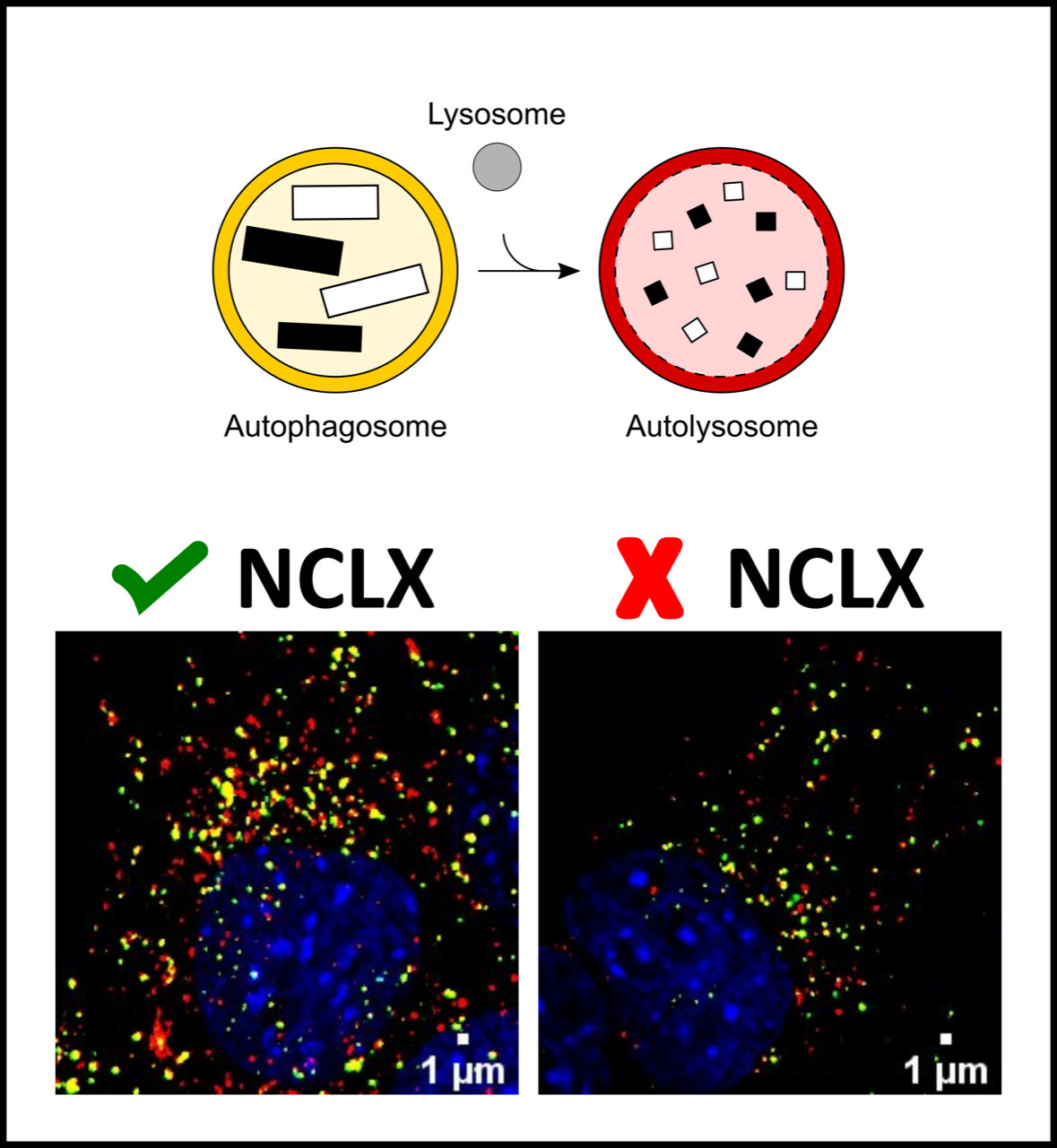
In parallel, they investigated what happened to cells in which there was less NCLX. For this purpose, in one experiment they reduced protein expression using the genetic knockdown (KD) technique of NCLX by transfection of small interfering RNAs (siRNA) and in another, they caused acute inhibition of NCLX activity through pharmacological means. In both cases, the researchers saw that NCLX decrease impairs basal and starvation-induced autophagy.
According to Kowaltowski, not all processes that regulate autophagy described in the literature affect basal autophagy. “The fact that it alters basal autophagy is very significant, and shows that NCLX is indeed an important regulator in the cell.“
Mechanism
From different measurements carried out at various stages of the autophagy process, researchers discovered that NCLX activity affects the initial steps of the autophagic machinery. In autophagy, cellular components are sequestered by autophagosomes, which in turn fuse with lysosomes to promote the digestion of organelles or proteins. With the data obtained in this study, the researchers concluded that the decrease in intracellular calcium resulting from NCLX inactivation interferes with the formation of the autophagosome, impairing the initial stages of autophagy.
Contrary to what was expected, the researchers also saw that the inhibition of NCLX did not alter the production of ATP in mitochondria, nor did it affect the AMP-activated protein kinase (AMPK) pathway, which is activated when energy is low in the cell. “This regulation of autophagy by NCLX is happening through cellular calcium, independent of the AMPK pathway,” concludes Kowaltowski.
The cell’s energy production is closely linked to autophagy. The lack of energy causes autophagy, which releases nutrients that the cell can use in that energetic emergency. In 2023, Kowaltowski’s group published a paper showing an optimal range of calcium in which mitochondrial respiration, and therefore energy production, is activated. Although NCLX inhibition altered the cell’s calcium concentration enough to affect autophagy, the mitochondrial calcium level did not leave the ideal range.
According to the researchers, additional studies are needed to elucidate the complex mechanisms controlling autophagy by cytoplasmic calcium. “Obviously, calcium signaling is very complex, perhaps promiscuous in the cell, as many proteins can bind to calcium and be regulated by it, so it is difficult to come up with a specific mechanism,” said Ramos.
The researcher carried out part of the study with a Research Internship Abroad Grant (BEPE) from FAPESP in the laboratory of Professor Viktor I. Korolchuk from Newcastle University in England. “They have expertise in autophagy and brought several tools to make different measurements and look at different aspects of autophagy.“
NCLX
The gene that encodes the NCXL transporter, a mitochondrial protein, was identified in 2010. Since then, according to the researchers, several studies have revealed important physiological functions NCLX promoted by shaping calcium signals, including the regulation of insulin secretion, self-stimulation of the cardiac muscle cell, and the secretion of lactate and glutamate in the astrocytes.
Decreased NCLX expression has been observed in some pathologies, such as Alzheimer’s disease and Parkinson’s disease. “It has been seen that patients who died with Alzheimer had less NCLX. In animal models, when researchers revert to normal NCLX levels, they can improve the disease phenotype,” says Ramos.
The brain uses a lot of energy and needs a lot of mitochondria. The accumulation of cell damage can lead to cell death and the development of these pathologies. “Although we did not look specifically at pathological contexts, this link between calcium signaling and autophagy is interesting because it could be an idea of mechanisms of progression in some diseases. By losing the activity of this transporter, more damage is generated, and the process that clearing this damage, autophagy, is impaired. This opens up perspectives for future work”, Kowaltowski said.
The article Mitochondrial Sodium/Calcium Exchanger (NCLX) Regulates Basal and Starvation-Induced Autophagy Through Calcium Signaling, by Vitor de Miranda Ramos, Julian D.C. Serna, Eloisa A. Vilas-Boas, João Victor Cabral-Costa, Fernanda M. da Cunha, Tetsushi Kataura, Viktor I. Korolchuk and Alicia J. Kowaltowski, can be accessed here.
